Ion-solvation is the important term that describes all type of ion-solvent interactions. The electrolytic solution contains an electrolyte in ionized form either completely or partially. The ionisation of an electrolyte in solution indicates the ion-solvation. The solubility of electrolytes are greatly affetced by the solvation of their constituent ions.
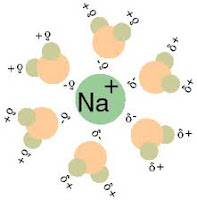
The term solvation refers to the surrounding of each dissolved molecule or ion by a shell of more or less tightly bound solvent molecules. This solvent shell is the result of intermolecular forces between solute and solvent. For aqueous solutions the term used is hydration. During the dissolution process, when a relatively small amount of solute dissolves in relatively large amount of solvent to form a homogeneous phase, a variety of intermolecular forces play a role such as solvent-solvent interaction, solute-solvent interaction and solute-solute interaction.
The study of ion-solvation in water and mixed solvents(water + organic) has proliferated in recent years because of its importance due to the availability of techniques and also its foreseeable applications. The technique that have been evolved mainly electrochemical and spectroscopic applications are for instance, in the field of high energy density batteries, hydrometallurgy, determination of simple ion Gibbs energy changes, in the preparation of model for polar clusters, in preparation of ion-selective PVC membrane electrodes in determination of structure of ion-solvation state of bimetallic salts and its derivatives in solvents, determination of bulk single ion solvation enthalpies , ion association of complexes in pure and mixed solvents etc
The term solvation refers to the surrounding of each dissolved molecule or ion by a shell of more or less tightly bound solvent molecules. This solvent shell is the result of intermolecular forces between solute and solvent. For aqueous solutions the term used is hydration. During the dissolution process, when a relatively small amount of solute dissolves in relatively large amount of solvent to form a homogeneous phase, a variety of intermolecular forces play a role such as solvent-solvent interaction, solute-solvent interaction and solute-solute interaction.
The study of ion-solvation in water and mixed solvents(water + organic) has proliferated in recent years because of its importance due to the availability of techniques and also its foreseeable applications. The technique that have been evolved mainly electrochemical and spectroscopic applications are for instance, in the field of high energy density batteries, hydrometallurgy, determination of simple ion Gibbs energy changes, in the preparation of model for polar clusters, in preparation of ion-selective PVC membrane electrodes in determination of structure of ion-solvation state of bimetallic salts and its derivatives in solvents, determination of bulk single ion solvation enthalpies , ion association of complexes in pure and mixed solvents etc
No comments:
Post a Comment